negative electron affinity trend|electron affinity across periods : Bacolod The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a . Elmo parts Price from €470 New and used Trusted sellers Currently in stock A great selection of spare parts at the Machineryline platformDo you consider your project as Environmental Enhancement? *All blue icons need to turn green before you can proceed to next step. The online screening module for ECC Requirement .
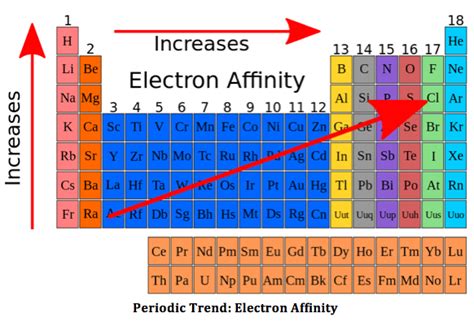
negative electron affinity trend,Electron affinity is a measure of the energy released when an extra electron is added to an atom. Electron affinities are measured in the gaseous state. In general, electron affinities become more negative as we move from left to right on the periodic table. In general, electron affinities become less . Tingnan ang higit pa

The energy change that occurs when a neutral atom gains an electron is called its electron affinity. When energy is released in a chemical reaction or . Tingnan ang higit paIn most cases, the formation of an anion by the addition of an electron to a neutral atom releases energy. This can be shown for the chloride ion . Tingnan ang higit paelectron affinity across periodsDefine “electron affinity." Does addition of an electron to a neutral atom require energy to be absorbed or released? Describe the general trend for . Tingnan ang higit pa The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a .negative electron affinity trendElectron affinity is the energy change that results from adding an electron to a gaseous atom. For example, when a fluorine atom in the gaseous state gains an electron to form . Electron Affinity Trend on the Periodic Table. Like electronegativity, ionization energy, atomic or ionic radius, and metallic character, electronegativity .Periodic trends (such as electronegativity, electron affinity, atomic and ionic radii, and ionization energy) can be understood in terms of Coulomb's law, which is Fₑ = (q₁q₂)/r².
The negative sign shows that the process releases energy. Adding an electron to a metal requires energy. Metals are much more likely to give up their electrons. Thus, metals have positive electron affinities. For .
As we travel down groups, electron affinities become more negative, meaning the process is more endothermic. Electron proximity to these respective nuclei also influences this phenomenon, but contrary to the .Electron affinity is the attraction a neutral atom has for a non-bonding electron. Moving from left to right and bottom to top on the period table, electron affinity increases. This is because going from left to right and .
The more negative the electron affinity value, the higher an atom's affinity for electrons. Figure \(\PageIndex{5}\): Periodic Table showing Electron Affinity Trend. Electron affinity generally decreases down a group of elements because each atom is larger than the atom above it (this is the atomic radius trend, discussed below).

The electron affinity also does not follow the trend from group 1 to 2, and 14 to 15, because the gain of an additional electron results in a less stable or more unfavorable electron configuration .
negative electron affinity trend electron affinity across periods The electron affinity also does not follow the trend from group 1 to 2, and 14 to 15, because the gain of an additional electron results in a less stable or more unfavorable electron configuration .Transcript. Electronegativity is a measure of an atom's ability to attract shared electrons to itself. On the periodic table, electronegativity generally increases as you move from left to right across a period and decreases as you move down a group. As a result, the most electronegative elements are found on the top right of the periodic table .Electron Affinity Trends. Electron Affinity is the energy associated with the addition of an electon to a gaseous atom. Example: Cl(g) + e- → Cl-(g) E.A. = -349 kJ/mol. Notice the sign on the energy is negative. This is because energy is usually released in this process, as opposed to ionization energy, which requires energy. And this amount of energy change (ΔE) is called electron affinity. This energy change (ΔE) can be positive, negative or zero. And the sign of Electron Affinity (E EA) is opposite to the sign of energy change (ΔE). E EA = – (ΔE) In simple words, if ΔE is positive, then E EA will be negative. And if ΔE is negative, then E EA will be positive.Electron affinities are the negative ion equivalent, and their use is almost always confined to elements in groups 6 and 7 of the Periodic Table. The first electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. This is more easily seen in symbol terms. The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom: E(g) +e− → E−(g) energy change=EA (1.1.2.4.1) (1.1.2.4.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is .Electron Affinity Trends. Electron Affinity is the energy associated with the addition of an electon to a gaseous atom. Example: Cl(g) + e- → Cl-(g) E.A. = -349 kJ/mol. Notice the sign on the energy is negative. This is because energy is usually released in this process, as opposed to ionization energy, which requires energy.
A negative electron affinity indicates that the process absorbs energy (it is endothermic), increasing the energy of the system. Electron affinities are normally expressed in kJ/mol. “Electron affinity” is often used to refer to the process itself (adding an electron to a gaseous atom): X () + e X () g g – –. The elements of the halogen group (Group 17) gain electrons most readily, as can be seen from their large negative electron affinities. This means that more energy is released in the formation of a halide ion than for the anions of any other elements. . Describe the general trend for electron affinity values moving from top to bottom in a . The electron affinity ( EA E A) of an element E E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: E(g) +e− → E−(g) energy change=EA (4.5.1) (4.5.1) E ( g) + e − → E ( g) − energy change= E A. Unlike ionization energies, which are always positive for a neutral atom because energy is .
Locate the elements in the periodic table. Use the trends in electron affinities going down a column for elements in the same group. Similarly, use the trends in electron affinities from left to right for .
Reduction is a reaction that results in the gaining of an electron. Reduction potentials follow the same trend as the electron affinity. That is because the larger, negative electron affinity, the easier it is to give an electron. Example of Reduction: \[F_{(s)} + e^- .For example, nonmetals like the elements in the halogens series in Group 17 have a higher electron affinity than the metals. This trend is described as below. Notice the negative sign for the electron affinity which shows that energy is released. Fluorine (F) -328 kJ mol-1; Chlorine (Cl) -349 kJ mol-1; Bromine (Br) -324 kJ mol-1; Iodine (I . The electron affinity of an element is defined as the energy released when an electron is added to an isolated gaseous atom to form a gaseous anion, or negative ion. Usually, only one electron is added, forming a uninegative ion. Since energy is evolved, these terms have a negative sign.Electron Affinity. The electron affinity (EA) of an element E is defined as the energy change that occurs when an electron is added to a gaseous atom or ion: [latex]E_{(g)}+e^- \rightarrow E^-_{(g)} \;\;\; \text{energy change=}EA \label{7.5.1}[/latex] Unlike ionization energies, which are always positive for a neutral atom because energy is required to .The chemical equation for electron affinity is as follows [1-4]. X (g) + e – → X – (g) When an electron approaches a neutral atom, the positively charged nucleus attracts it. The atom transforms into a negatively charged ion or an anion. Consequently, energy is released in this process, making the process exothermic.
negative electron affinity trend|electron affinity across periods
PH0 · what is electron affinity in chemistry
PH1 · trend of electron affinity in periodic table
PH2 · group 1a lowest electron affinity
PH3 · electron affinity vs electronegativity
PH4 · electron affinity trends explained
PH5 · electron affinity trend and exceptions
PH6 · electron affinity chart
PH7 · electron affinity across periods
PH8 · Iba pa